Modification of Antibody Disulfide Bonds with Maleimides
Abstract
In the last two decades antibody based applications have found their way into many areas of the life sciences as well as the clinic due to an increased understanding of their function coupled to a huge progress in the techniques for their manipulation and engineering. The high specificity, selectivity and affinity of the antibody-antigen interaction makes these proteins perfectly suited to locate and visualise or neutralise their target in most biological backgrounds and various experts in the field expect antibodies to be the core principle of the next wave of novel therapeutics.
To broaden their scope and versatility antibodies are often chemically modified with functional groups such as fluorophores, radio labels or polymers. The challenge here lies in the preservation of the binding activity as most chemically methods offer only poor site-specificity (e.g. lysine modification) with the conjugation potentially occurring in the CDR region and the widely used site-specific method of cysteine genetic engineering is hampered by interference with the complex disulfide structure of antibodies.
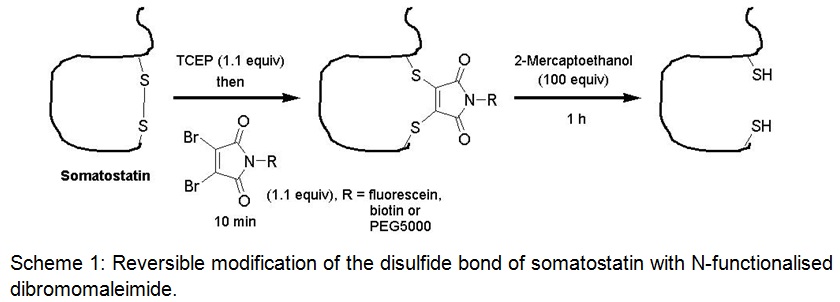
We have recently demonstrated that 3,4-substituted maleimides can be used to functionalise disulfide bonds with high selectivity (Scheme 1) and have also established an in situ protocol for this reaction which would be necessary to retain the correct cystine network of the target antibody. We were now able to show that this methodology is suitable to access the disulfide bond of an anti-carcinoembryonic antigen (CEA) scFv antibody fragment which was hereupon conjugated to fluorescein, biotin or a 5 kDa PEG chain and full binding activity of the modified molecule was demonstrated by ELISA and Biacore assays. Further N-substituted dithiophenolmaleimides were employed to spin label various isotypes of commercially available anti-FLAG antibodies via their disulfide bonds and again full activity was retained as shown by ELISA. These proof-of-principal studies suggest a broad applicability of the maleimide technology for the site-specific modification of antibodies.
References
Tedaldi et al. (2009) Bromomaleimides: new reagents for the selective and reversible modification of cysteine. Chem. Comm., 6583-5.
Smith et al. (2010) Protein Modification, Bioconjugation and Disulfide Bridging Using Bromomaleimides. J. Am. Chem. Soc., 132, 1960-5.
Schumacher et al. (2011) In Situ Maleimide Bridging of Disulfides and a New Approach to Protein PEGylation. Bioconjugate Chem., 22, 132-6.
Ryan et al. (2011) Tunable reagents for multi-functional bioconjugation: reversible or permanent chemical modification of proteins and peptides by controlof maleimide hydrolysis. Chem. Comm., 5452-4.

