Cyclolisation: The last step in the biosynthesis of D-lysergic acid alkaloids
Abstract
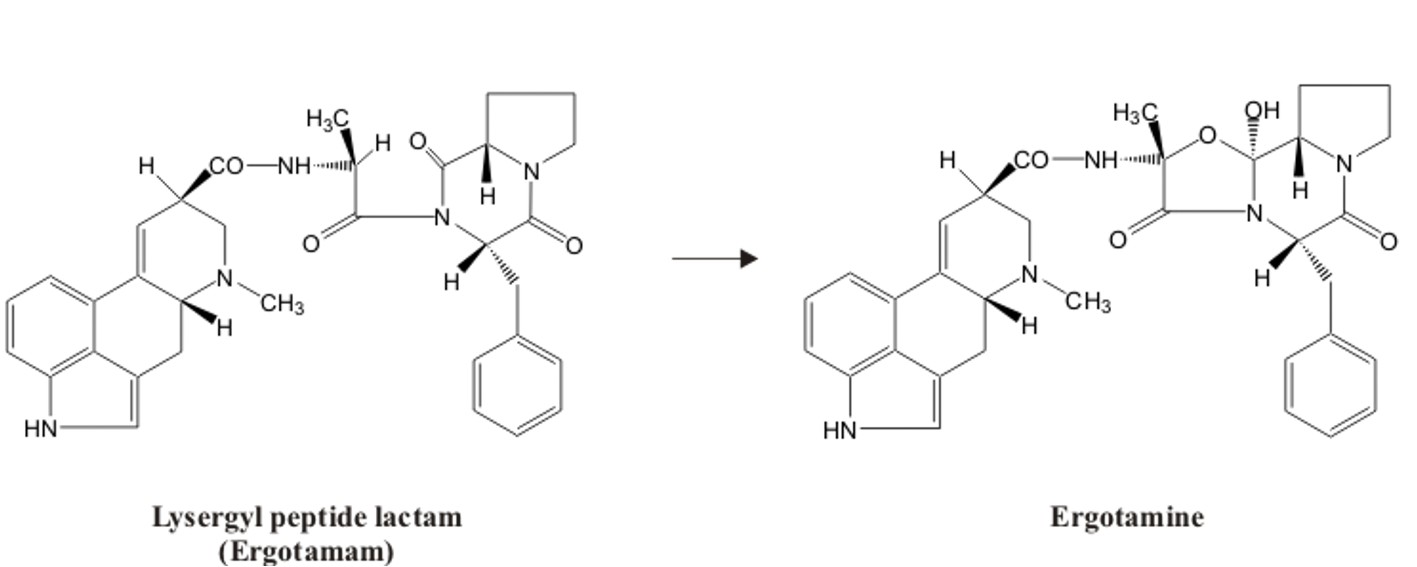
The ergopeptines, alkaloid cyclopeptides from ergot fungi, contain a unique cyclol structure which results from lactonization of a hydroxyacyl lactam with the generation of a tertiary hydroxyl group. Ergopeptines are so-called oxacyclols which are formed when a hydroxyl group is added to a peptide carbonyl group (1). For long it was unclear how this last step in the biosynthesis of the ergopeptines occurs. It was postulated, that a monoxygenase, possibly a P450 monooxygenase, catalyzes this reaction via hydroxylation (2, 3). However, this was based solely on in vivo observations.
Recently, we detected in enzyme fractions from Claviceps purpurea which contain the nonribosomal peptide synthetases LPS1 and LPS2 a new enzyme activity that converts D-lysergyl-alanyl-phenylalanyl-proline lactam into ergotamine by α-hydroxylation of the amino acid adjacent to D-lysergic acid.
The enzyme was partially purified and characterized in respect of substrate specificity and kinetic constants. Partial sequencing of the protein is in progress to obtain sequences suitable for deriving probes which will enable us to clone the gene.
References
(1) Hofmann et al. (1963) "Synthese von Ergotamin". Helvetica Chimica Acta 46: 2306–2336
(2) Belzecki et al. (1980) Mechanism of α-hydroxy-α-amino acid formation in the biosynthesis of peptide ergot alkaloids. J. Org. Chem. 45: 2215-2217
(3) Quigley and Floss (1981) Mechanism of amino acid α-hydroxylation and formation of the lysergyl moiety in ergotamine biosynthesis. J. Org. Chem. 46: 464-466

