Highly efficient and more general cis- and trans-splicing inteins through sequential directed evolution
Abstract
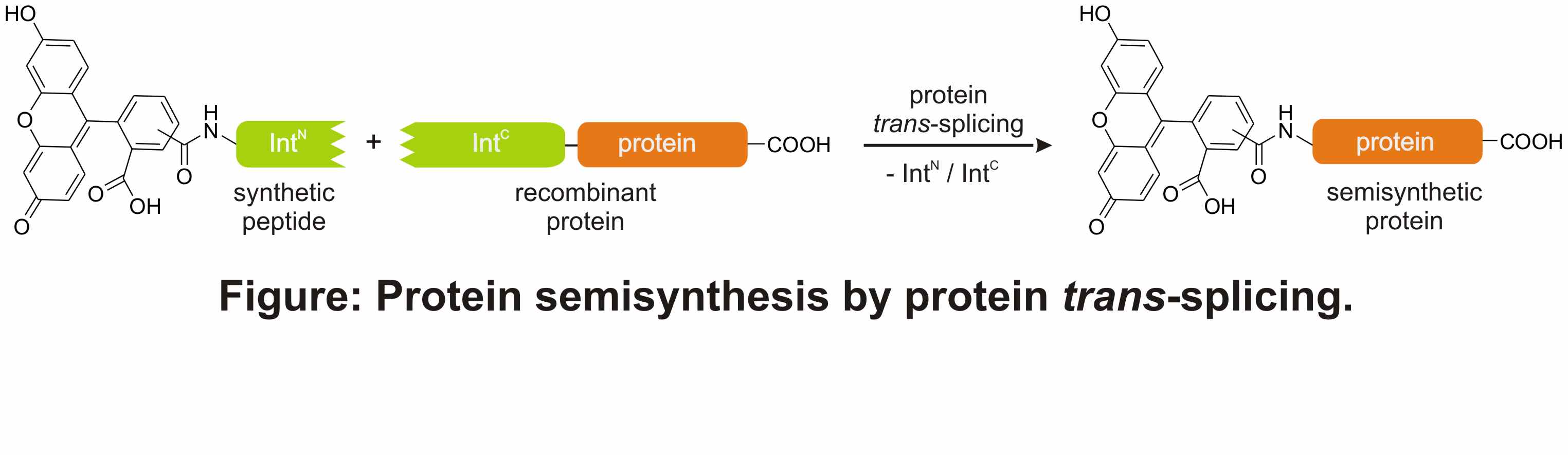
Chemoenzymatic approaches bear potential advantages over chemical ligation methods in the generation of site-selectively modified proteins. Two polypeptide segments, of which one is synthetically and the other recombinantly produced, can be linked through protein trans-splicing to give a semisynthetic protein (see Figure). The Ssp DnaB intein was previously shown to support protein trans-splicing when split following aa 11 (S1-site).1 Taking advantage of the short IntN segment it was established as an attractive tool for ligating synthetic peptide fragments to the N terminus of proteins.2,3
We here report the biochemical characterization of an evolved mutant of the Ssp DnaB intein.4 The trans-splicing activity of the mutant was investigated in the context of a model protein and compared to the parent intein. Strikingly, the first-order rate of trans-splicing was increased ~60-fold, such that about 90% splice product formation is achieved within 30 min with negligible formation of the C-terminal cleavage by-product. Additionally, the evolved mutant showed high splicing activity with a non-native extein residue at the (-1) position, for which the parent intein was almost inactive.4
Thus, even though the mutations were selected in the context of a cis-intein under cellular expression conditions in E. coli, they also dramatically improved the splicing capability of the artificially split intein under in vitro conditions.
References
1. Sun, W. et. al., J. Biol. Chem. 279, 35281-35286 (2004).
2. Ludwig, C. et. al., Angew. Chem. Int. Ed. Engl. 45, 5218-5221 (2006).
3. Ludwig, C. et. al., J. Biol. Chem. 283, 25264-25272 (2008).
4. Liu, X.Q., Mootz, H. D. et. al., submitted.

