Studies on the chemical structure requirements of a semisynthetic split intein
Abstract
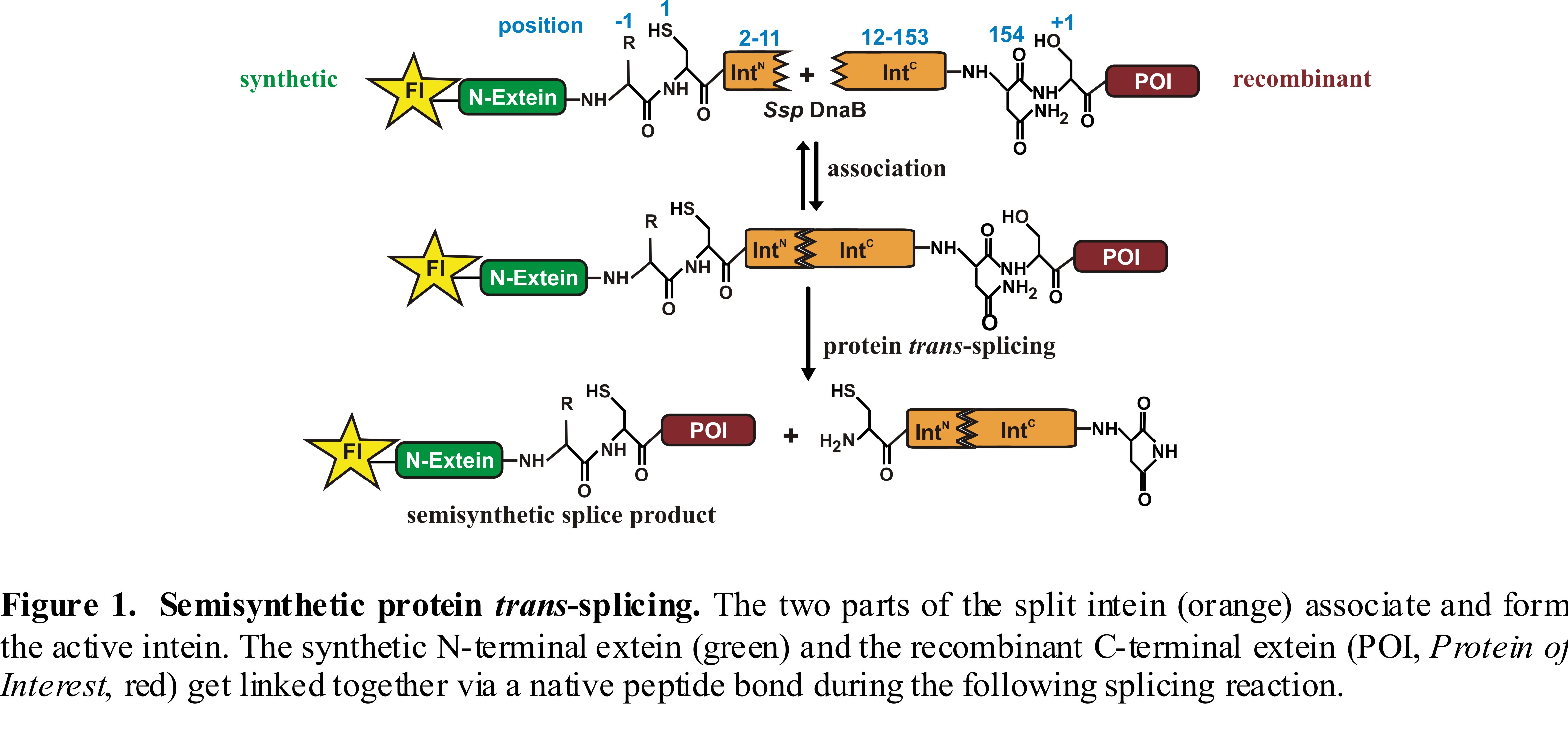
An intein is an internal protein sequence that undergoes protein splicing by excising itself out of a precursor protein, with concomitant linking of the flanking amino acid sequences, termed the N- and C-exteins, with a native peptide bond. In the case of protein trans-splicing, the intein is split into two parts which have to reconstitute before the splicing reaction can occur. If the intein is used to ligate a synthetic and a recombinant fragment the reaction is called semisynthetic protein trans-splicing [1]. Regarding the tremendous potential for the use of inteins in protein semi-synthesis, the tolerance towards the nature of the extein sequences is of great interest. However, it is not well understood which amino acids of the exteins near the N- and C-terminal splice junction are essential or variable and what the molecular origins for this dependence are.
To find out more about the structural and functional features of the N-extein sequence the Ssp DnaB intein split at position 11 was used [2,3]. The advantage of this intein is the short N-terminal part which is easily synthesized via solid phase peptide synthesis while the C-terminal half is generated by recombinant protein expression. In the case of this intein, the amino acid glycine at position -1 (the first amino acid N-terminal to the intein) is of special importance for the activity of the intein and studies showed that a mutation of this residue almost completely inhibits the splice activity [4]. By using chemical peptide synthesis, we dissect which structural changes in the side chain and backbone structures of the amino acid (-1) are tolerated. We also compare these findings with an evolved mutant of the Ssp DnaB intein that shows more robust splicing activity. Furthermore mechanistic studies using this semisynthetic intein will be presented. Our results have useful implications for the preparation of semisynthetic proteins by the split intein approach.
References
1. Noren, C.J., Wang, J., Perler, F.B., Angew. Chem. 2000, 112,458-76
2. Sun,W., Yang, J., Liu, X., J.Biol.Chem. 2004, 279(34), 35281-86
3. Ludwig, C., Pfeiff, M., Linne, U., Mootz, H.D., Angew. Chem. 2006, 45, 5218-21
4. Ludwig, C., Schwarzer, D., Mootz, H.D., J.Biol.Chem. 2008, 283(37), 25264-72

