Inclusion body display: in vivo protein immobilization for applications in biochemistry and biotechnology
Abstract
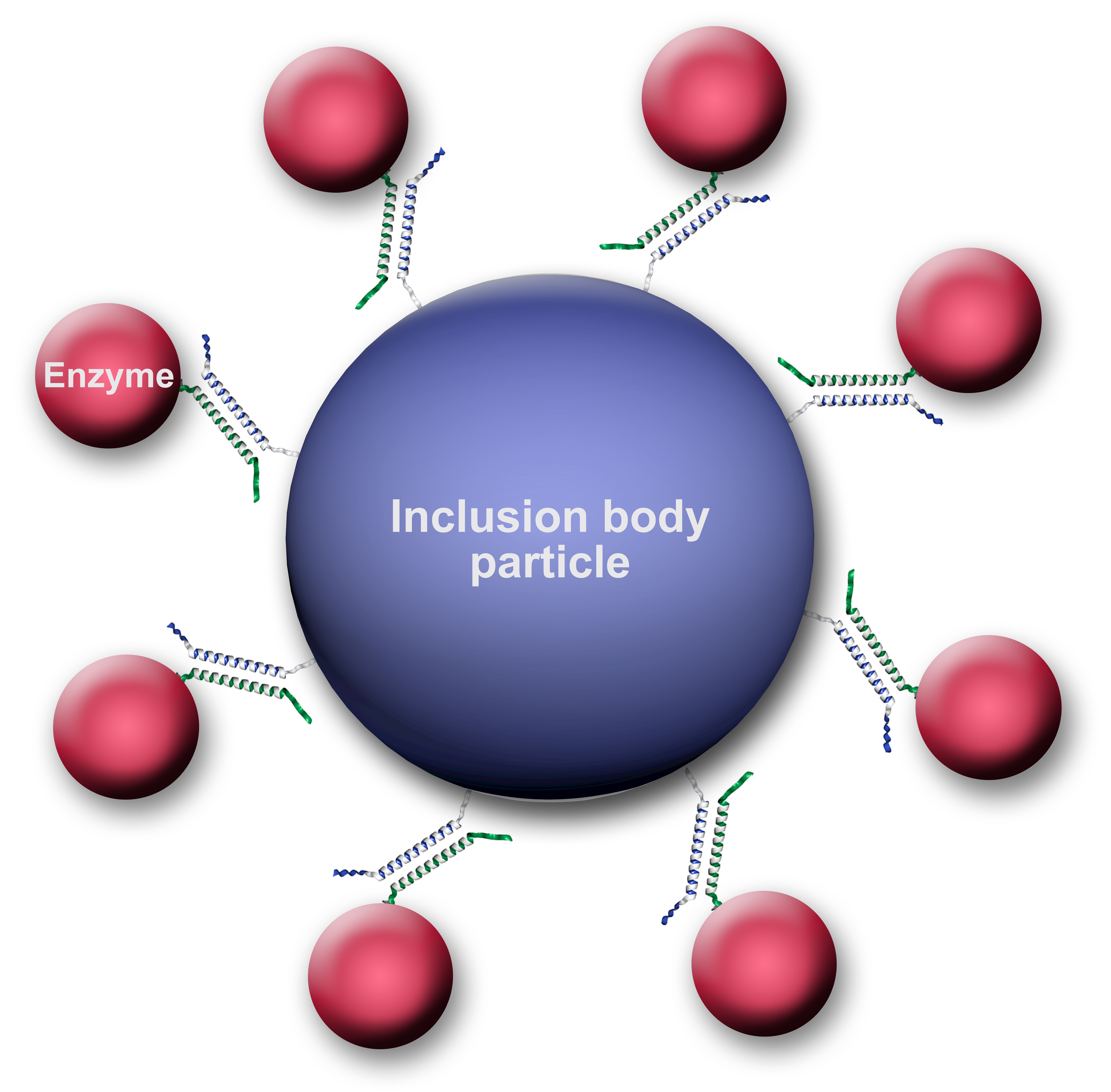
We have established a novel strategy for in vivo immobilization of active enzymes on the surface of inclusion body particles. It relies on expression in E. coli of polyhydroxybutyrate synthase PhaC from Cupriavidus necator, which contains an engineered negatively charged α-helical coil (Ecoil) and forms inclusion bodies upon high-level expression. Coexpression in the same cell of a desired enzyme carrying a positively charged coil (Kcoil) sequence results in heterodimeric coiled-coil formation in vivo and in the capture of the enzyme in active form on the surface of the inclusion body particle, which can easily be isolated from lysed cells simply by centrifugation (Fig.1).[1] To show full enzymatic activity of immobilized enzymes and to demonstrate the ability for analyzing the functionalized particels by flow cytometry, we successfully immobilized galactose oxidase from Fusarium spp.[2] Additionally we developed a modular system of immobilized biocatalysts providing a full cofactor regeneration cycle enabling the stereoselective conversion of asymmetric ketones to chiral alcohols. This NADH-dependent binary system includes two enzymes, glucose dehydrogenase from B. subtilis and alcohol dehydrogenase from R. erythropolis, each immobilized separately on inclusion body particles.[3] The cost-effective one-step generation and isolation of enzymes immobilized on inclusion body particles may become useful for various applications in bioprocessing and biotransformation.
References
[1] Steinmann et al. (2010) Appl Environ Microbiol. 76(16):5563-5569.
[2] Escalettes and Turner (2008) Chembiochem.9(6):857-860.
[3] Gröger et al. (2009) Angew. Chem. 118:5806 -5809.

