Structural basis of flocculin-mediated adhesion in yeast
Abstract
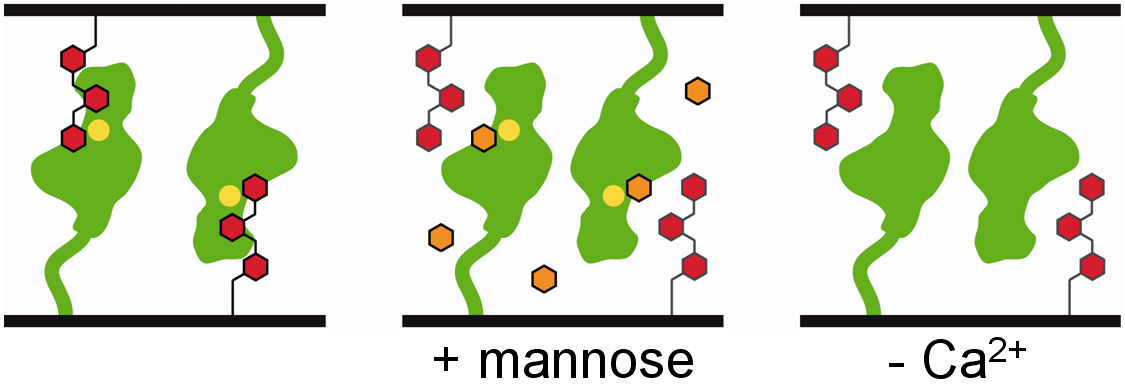
Saccharomyces cerevisiae is able to undergo a variety of vegetative adhesion-dependent developmental processes including cell-cell adhesion to form flocs or cell-substrate adhesion for biofilm and filament formation. For this purpose, S. cerevisiae makes use of a family of cell-surface flocculins, that have a common architecture. These proteins consist of an N-terminal A domain thought to confer adhesion-specific properties, a B domain that comprises highly glycosylated repeats and a C domain providing cell wall anchoring. Previous studies have shown that flocculation is mediated by the highly related Flo1, Flo5 and Flo9 proteins, whereas Flo11 confers cell-surface adhesion[1]. We obtained several complex structures of Flo5A, the N-terminal binding domain of Flocculin-5, in combination with biochemical studies on its carbohydrate specificity. The structure was determined at an atomic resolution of 0.95 Å and revealed an unusual calcium binding motif, the DcisD motif, which proved to be essential for carbohydrate-binding activity[2]. Furthermore, an interesting observation was made, when phasing experiments were conducted. Due to a distinct conformation of four acidic residues on the protein surface, the formation of a lanthanoid cluster consisting of seven gadolinium atoms and bridging water could be observed. This cluster is compatible with known small molecule structures and could aid in the development of novel MRI contrast agents based on protein scaffolds.
References
[1] Verstrepen, K, et al., (2006), Mol Microbiol, 60, 5
[2] Veelders, M, et al., (2010), Proc Natl Acad Sci USA, 107, 22511

